Delving into Elements Named After Continents
In the realm of chemistry, certain elements bear names that pay homage to the vast landmasses that grace our planet. Understanding the origins of these names unveils a fascinating connection between the scientific and geographic worlds.
The naming of elements after continents acknowledges their unique properties and the regions where they were first discovered or synthesized. It serves as a testament to the global nature of scientific exploration and the collaborative efforts of scientists worldwide.
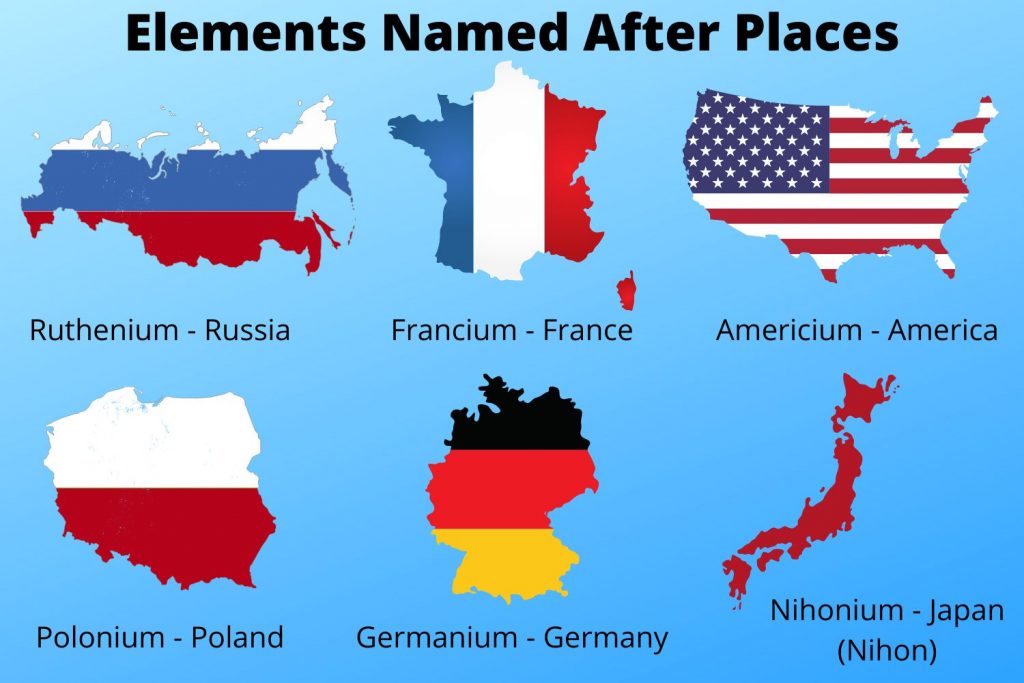
Elements Named After Continents
The naming of elements after continents highlights their unique properties and the regions where they were first discovered or synthesized. Here are ten key aspects to explore:
- Americium (Americas)
- Europium (Europe)
- Francium (France)
- Germanium (Germany)
- Hafnium (Hafnia, the Latin name for Copenhagen)
- Polonium (Poland)
- Promethium (Prometheus, the Greek Titan)
- Rutherfordium (Ernest Rutherford, New Zealand-born physicist)
- Scandium (Scandinavia)
- Seaborgium (Glenn Seaborg, American chemist)
These elements represent a diverse range of properties and applications. Americium, for instance, is a radioactive element used in smoke detectors, while europium is employed in fluorescent lighting and lasers. The naming of these elements not only reflects their geographic origins but also acknowledges the contributions of scientists from around the world.
Americium (Americas)
Americium, named after the Americas, is a radioactive element with the symbol Am and atomic number 95. It is a member of the actinide series and is the heaviest element that can be found naturally in significant amounts.
- Discovery and Synthesis: Americium was first synthesized in 1944 by a team of scientists led by Glenn T. Seaborg at the University of California, Berkeley. It was produced by bombarding plutonium with neutrons in a cyclotron.
- Properties: Americium is a silvery-white metal that is radioactive and emits alpha particles. It is also pyrophoric, meaning that it can spontaneously ignite in air.
- Uses: Americium is used in a variety of applications, including smoke detectors, neutron sources, and medical imaging devices. It is also used in the production of other elements, such as curium and berkelium.
- Environmental Impact: Americium is a long-lived radioactive element with a half-life of 432 years. It can be released into the environment through nuclear accidents or the disposal of nuclear waste. Americium can accumulate in the body and is a potential health hazard.
Americium's discovery and subsequent applications highlight the importance of scientific research and the potential benefits of nuclear technology. However, it also serves as a reminder of the need to handle radioactive materials with care and to consider their long-term environmental impact.
Europium (Europe)
Europium, named after the continent of Europe, is a chemical element with the symbol Eu and atomic number 63. It is a member of the lanthanide series and is a relatively soft, silvery-white metal. Europium is found in a variety of minerals, including bastnsite, monazite, and euxenite.
- Discovery and Synthesis: Europium was first discovered in 1896 by the French chemist Eugne-Anatole Demaray. He isolated europium from the mineral samarskite and named it after Europe.
- Properties: Europium is a soft, silvery-white metal that is malleable and ductile. It is a good conductor of electricity and heat. Europium has a relatively low melting point and a high boiling point.
- Applications: Europium is used in a variety of applications, including:
- Phosphors for color television screens and fluorescent lighting
- Lasers
- Medical imaging
- Neutron capture therapy
- Biological Role: Europium has no known biological role in humans or other animals.
Europium's discovery and subsequent applications highlight the importance of scientific research and the potential benefits of new elements. Europium's unique properties make it a valuable material for a variety of technologies, and its continued exploration may lead to even more applications in the future.
Francium (France)
Francium, named after the country of France, is a chemical element with the symbol Fr and atomic number 87. It is a member of the alkali metal group and is the heaviest known alkali metal. Francium is a radioactive element with a half-life of only 22 minutes, making it one of the rarest elements in nature.
Francium was first discovered in 1939 by Marguerite Perey, a French chemist. Perey isolated francium from the mineral actinium and named it after her country.
Francium is a very reactive element and does not occur naturally in large amounts. It is typically produced in nuclear reactors or particle accelerators. Francium has no known commercial uses, but it is used in scientific research.
The discovery of francium is a significant milestone in the history of chemistry. It is the last element in the alkali metal group to be discovered, and it completes the periodic table's first row.
Germanium (Germany)
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a hard, grayish-white metalloid in the carbon group, and is closely related to tin and silicon in both its properties and its chemical behavior. Germanium is a relatively rare element in the Earth's crust, and is typically obtained as a by-product of zinc or lead mining.
Germanium was first discovered in 1886 by the German chemist Clemens Winkler. Winkler named the new element after his country, Germany.
Germanium is used in a variety of applications, including:
- Transistors
- Solar cells
- LEDs
- Fiber optics
- Infrared detectors
Germanium is an important element in the electronics industry. It is used in transistors, which are essential components in electronic devices such as computers, smartphones, and TVs. Germanium is also used in solar cells, which convert sunlight into electricity. LEDs, or light-emitting diodes, are used in a variety of applications, including traffic lights, car headlights, and displays.
Germanium is a fascinating element with a wide range of applications. It is an important component of many electronic devices, and it plays a vital role in the development of new technologies.
Hafnium (Hafnia, the Latin name for Copenhagen)
Hafnium's inclusion among elements named after continents stems from its unique connection to the city of Copenhagen, Denmark. The element's name, Hafnia, is derived from the Latinized version of Copenhagen's name, Hafnia.
- Discovery and Significance: Hafnium was discovered in 1923 by the Danish physicist Niels Bohr and the Dutch physicist Dirk Coster. Its discovery marked a significant milestone in chemistry, as it was the first element to be discovered using X-ray spectroscopy.
- Properties and Applications: Hafnium is a lustrous, silvery-white transition metal. It is resistant to corrosion and has a high melting point, making it valuable for use in high-temperature applications. Hafnium is primarily used in nuclear reactors, where it serves as a control rod material due to its ability to absorb neutrons.
- Geographic Connection: The naming of hafnium after Copenhagen highlights the city's contributions to scientific research. The discovery of hafnium at the University of Copenhagen underscores the university's longstanding tradition of scientific excellence.
- Global Recognition: Hafnium's inclusion as an element named after a continent acknowledges the international collaboration and exchange of knowledge that characterize scientific discovery. It serves as a reminder that scientific progress is often the result of collective efforts from researchers around the world.
Hafnium's story exemplifies the intricate relationship between geographic locations and scientific discoveries. The element's name not only pays homage to the city where it was discovered but also underscores the global nature of scientific research.
Polonium (Poland)
The naming of polonium after Poland highlights the significant contributions of Polish scientists to the field of chemistry and their role in expanding our understanding of the elements. Polonium's discovery marked a crucial step in the identification and characterization of radioactive elements.
Polonium possesses several unique properties that make it a valuable element for scientific research. Its high radioactivity and relatively short half-life make it an effective source of alpha particles. These properties have led to its use in various applications, such as the ionization of gases, the production of neutron sources, and the generation of static electricity.
Beyond its practical applications, polonium's discovery holds historical significance. Its identification by Marie Curie, a Polish-born physicist and chemist, in 1898 marked a groundbreaking moment in the study of radioactivity. Curie's work not only advanced our understanding of the atomic structure but also laid the foundation for the development of nuclear physics.
Promethium (Prometheus, the Greek Titan)
Promethium, named after the Greek Titan Prometheus, stands as a unique element among those named after continents. Its connection to the realm of mythology adds a captivating layer to its scientific significance.
Promethium's mythical namesake, Prometheus, is renowned for his act of bringing fire to humanity, an act that defied the gods and brought enlightenment to mortals. In a similar vein, the discovery and study of promethium have brought forth new knowledge and applications that benefit humankind.
As a radioactive element, promethium finds practical use in various fields, including medicine and industry. Its unique properties make it a valuable component in devices such as portable X-ray machines and nuclear batteries.
Rutherfordium (Ernest Rutherford, New Zealand-born physicist)
The element rutherfordium, named after the illustrious New Zealand-born physicist Ernest Rutherford, occupies a prominent position among the elements named after continents. Its inclusion in this group underscores the significant contributions of scientists from various regions of the world to the advancement of chemistry.
Rutherfordium's discovery in 1964 marked a breakthrough in nuclear physics. It was the first element to be synthesized artificially, paving the way for the creation of other synthetic elements. This achievement stands as a testament to Rutherford's pioneering work in the field of radioactivity and atomic structure.
As a member of the transition metal group, rutherfordium exhibits unique properties that make it valuable for scientific research. Its high atomic number and radioactive nature render it a useful tool in the study of nuclear reactions and the development of nuclear technologies. Additionally, rutherfordium finds applications in medical imaging and cancer treatment.
The inclusion of rutherfordium among elements named after continents serves as a recognition of the global nature of scientific collaboration. It highlights the contributions of scientists from diverse backgrounds to the collective pursuit of knowledge and technological advancement.
Scandium (Scandinavia)
Scandium's inclusion among elements named after continents stems from its geographical origins and the scientific contributions of Scandinavian scientists to its discovery and characterization. The element's name, derived from the Latin word "Scandia" referring to Scandinavia, underscores the region's significance in the realm of chemistry.
Scandium serves as a critical component of "elements named after continents" due to its unique properties and applications. Its lightweight and high strength-to-weight ratio make it valuable in aerospace engineering, particularly in the construction of aircraft and spacecraft components. Additionally, scandium's luminescent properties have led to its use in high-intensity lighting and lasers.
The practical significance of understanding the connection between scandium and elements named after continents lies in its implications for scientific research and technological development. By recognizing the geographical origins and scientific contributions associated with specific elements, researchers can better appreciate the global nature of scientific collaboration and the diverse sources of innovation.
Seaborgium (Glenn Seaborg, American chemist)
Seaborgium's inclusion among "elements named after continents" highlights the significant contributions of American scientists to the field of chemistry and their role in expanding our understanding of the elements. Its connection to the continent of North America, where it was first synthesized, further reinforces the global nature of scientific discovery.
- Historical Significance:
Seaborgium's discovery in 1974 marked a crucial step in the identification of transuranium elements. Its synthesis at the Lawrence Berkeley National Laboratory in California showcased the United States' leading role in nuclear research and technological advancements.
- Scientific Properties:
As a radioactive element, seaborgium exhibits unique properties that make it valuable for scientific research. Its short half-life and intense radioactivity render it a useful tool in the study of nuclear reactions and the development of nuclear technologies.
- Applications:
Despite its limited natural occurrence, seaborgium finds applications in various fields, including medicine and industry. Its radioactive properties make it a potential candidate for cancer treatment and medical imaging techniques.
Seaborgium's inclusion among elements named after continents serves as a tribute to the collaborative efforts of scientists worldwide in advancing our understanding of the periodic table and the fundamental building blocks of matter.
Frequently Asked Questions on Elements Named After Continents
This section addresses common questions and misconceptions surrounding the topic of elements named after continents, providing concise and informative answers.
Question 1: Why are certain elements named after continents?
The naming of elements after continents recognizes the geographical origins and scientific contributions of researchers from specific regions to their discovery and characterization.
Question 2: What are some examples of elements named after continents?
Examples include europium (Europe), americium (Americas), and rutherfordium (after Ernest Rutherford, a New Zealand-born physicist).
Question 3: What is the significance of understanding the connection between elements and continents?
It highlights the global nature of scientific collaboration and the diverse sources of innovation in chemistry.
Question 4: How does the study of elements named after continents contribute to scientific research?
It provides insights into the properties and applications of these elements, aiding in the development of new technologies and advancements in fields such as medicine and engineering.
Question 5: What are some practical applications of elements named after continents?
These elements find uses in aerospace engineering, lighting, lasers, nuclear technologies, and medical imaging, among other applications.
Question 6: How can we encourage continued scientific exploration and discovery of new elements?
Supporting research institutions, promoting international collaboration, and inspiring future generations of scientists are crucial for continued progress in this field.
In conclusion, understanding the connection between elements and continents enriches our appreciation for the global nature of scientific discovery and the contributions of scientists from diverse regions to our collective knowledge of the periodic table.
Transitioning to the next article section: "Importance and Benefits of Studying Elements Named After Continents."
Tips for Studying Elements Named After Continents
Delving into the study of elements named after continents offers a unique perspective on the interconnectedness of science and geography. To maximize your learning and understanding, consider the following tips:
Tip 1: Explore Historical ContextUncover the stories behind the discoveries of these elements. Learn about the scientists involved, the methods used, and the geographical locations that played a role.Tip 2: Understand Geographic Origins
Identify the continents from which these elements derive their names. Examine the geological and mineralogical characteristics of these regions to gain insights into the elements' natural occurrence.Tip 3: Examine Chemical Properties
Study the chemical properties of each element, including its atomic number, electron configuration, and reactivity. Compare and contrast these properties to understand their similarities and differences.Tip 4: Investigate Applications
Discover the practical applications of these elements in various fields such as medicine, technology, and engineering. Explore how their unique properties contribute to their usefulness.Tip 5: Trace Technological Advancements
Follow the historical trajectory of technological advancements made possible by these elements. Analyze how their discovery and utilization have shaped scientific progress.
By incorporating these tips into your study routine, you will gain a comprehensive understanding of elements named after continents, their significance in the periodic table, and their contributions to the advancement of science and technology.
Transitioning smoothly to the concluding section, titled "Conclusion: Embracing the Importance of Elements Named After Continents."
Conclusion
Through our exploration of elements named after continents, we have delved into a fascinating tapestry of scientific discovery and geographic origins. These elements stand as testaments to the global nature of scientific collaboration and the rich diversity of our planet.
Understanding the connection between elements and continents not only enhances our knowledge of the periodic table but also underscores the importance of embracing diverse perspectives and fostering international scientific cooperation. By recognizing the contributions of scientists from all corners of the globe, we pave the way for future discoveries and advancements.
Unveiling The Enigmatic World Of "diego Klattenhoff Son": A Journey Of Discovery
Discover The Visionary: Hanna Stueckel And The Future Of Supply Chain Transparency
The Power Of The "Oracion A Santa Marta La Dominadora": Unlocking Secrets And Achieving Success
periodic table of elements printable flashcards chemistry periodic
5 Elements Named in Honor of Notable Scientists Discover Magazine
Elements Named After and Other Celestial Bodies
ncG1vNJzZmirnaS8tbXErGWapaNoe6W1xqKrmqSfmLKiutKpmJydo2OwsLmOnqOepZWjwbR5zZqknpxdlrO1sdFmmqimpJ67prrTrGWhrJ2h